Amino Acids
Amino acids are the building blocks of protein . The body has twenty different amino acids that act as these building blocks. Nonessential amino acids are those that the body can synthesize for itself, provided there is enough nitrogen, carbon, hydrogen, and oxygen available. Essential amino acids are those supplied by the diet , since the human body either cannot make them at all or cannot make them in sufficient quantity to meet its needs. Under normal conditions, eleven of the amino acids are nonessential and nine are essential.
Structure
All amino acids have a similar chemical structure—each contains an amino group (NH 2 ), an acid group (COOH), a hydrogen atom (H), and a distinctive side group that makes proteins more complex than either carbohydrates or lipids . All amino acids are attached to a central carbon atom (C).
The distinctive side group identifies each amino acid and gives it characteristics that attract it to, or repel it from, the surrounding fluids and other amino acids. Some amino acid side groups carry electrical charges that are attracted to water molecules (hydrophilic), while others are neutral and are repelled by water (hydrophobic). Side-group characteristics (shape, size, composition, electrical charge, and pH ) work together to determine each protein's specific function.
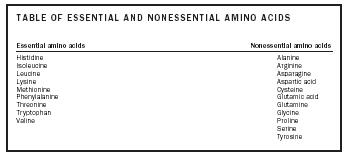
| Essential amino acids | Nonessential amino acids |
| Histidine | Alanine |
| Isoleucine | Arginine |
| Leucine | Asparagine |
| Lysine | Aspartic acid |
| Methionine | Cysteine |
| Phenylalanine | Glutamic acid |
| Threonine | Glutamine |
| Tr yptophan | Glycine |
| Valine | Proline |
| Serine | |
| Tyrosine |
![A diabetic child injects herself with insulin. Composed of 51 amino acids, insulin is a small protein used by the body to regulate glucose levels in the blood. [Custom Medical Stock Photo. Reproduced by Permission.]](../images/nwaz_01_img0024.jpg)
The three-dimensional shape of proteins is derived from the sequence and properties of its amino acids and determines its function and interaction with other molecules. Each amino acid is linked to the next by a peptide bond, the name given to the link or attraction between the acid (COOH) end of one amino acid and the amino end (NH 2 ) of another. Proteins of various lengths are made when amino acids are linked together in this manner. A dipeptide is two amino acids joined by a peptide bond, while a tripeptide is three amino acids joined by peptide bonds.
The unique shapes of proteins enable them to perform their various tasks in the body. Heat, acid, or other conditions can disturb proteins, causing them to uncoil or lose their shape and impairing their ability to function. This is referred to as denaturation.
Functions of Proteins
Proteins act as enzymes , hormones , and antibodies . They maintain fluid balance and acid and base balance. They also transport substances such as oxygen, vitamins , and minerals to target cells throughout the body. Structural proteins, such as collagen and keratin, are responsible for the formation of bones, teeth, hair, and the outer layer of skin, and they help maintain the structure of blood vessels and other tissues. In contrast, motor proteins use energy and convert it into some form of mechanical work (e.g., dividing cells, contracting muscle).
Enzymes are proteins that facilitate chemical reactions without being changed in the process. The inactive form of an enzyme is called a proenzyme. Hormones (chemical messengers) are proteins that travel to one or more specific target tissues or organs, and many have important regulatory functions. Insulin , for example, plays a key role in regulating the amount of glucose in the blood. The body manufactures antibodies (giant protein molecules), which combat invading antigens. Antigens are usually foreign substances such as bacteria and viruses that have entered the body and could potentially be harmful. Immunoproteins, also called immunoglobulins or antibodies, defend the body from possible attack by these invaders by binding to the antigens and inactivating them.
Proteins help to maintain the body's fluid and electrolyte balance. This means that proteins ensure that the proper types and amounts of fluid and minerals are present in each of the body's three fluid compartments. These fluid compartments are intracellular (contained within cells), extracellular (existing outside the cell), and intravascular (in the blood). Without this balance, the body cannot function properly.
Proteins also help to maintain balance between acids and bases within body fluids. The lower a fluid's pH, the more acidic it is. Conversely, the higher the pH, the less acidic the fluid is. The body works hard to keep the pH of the blood near 7.4 (neutral). Proteins also act as carriers, transporting many important substances in the bloodstream for delivery throughout the body. For example, a lipoprotein transports fat and cholesterol in the blood.
Food Sources
Humans consume many foods that contain proteins or amino acids. One normally need not worry about getting enough protein or amino acids in the typical American diet. Foods from animal sources are typically rich in essential amino acids. These include chicken, fish, eggs, dairy products, beef, and pork. With the increasing emphasis on vegetarian diets, plant sources of protein are gaining in popularity. Such sources include dried beans (black, kidney, northern, red, and white beans), peas, soy, nuts, and seeds. Although plant sources generally lack one or more of the essential amino acids, when combined with whole grains such as rice, or by eating nuts or seeds with legumes , all the amino acids can be obtained.
SEE ALSO Diet ; Fats ; Malnutrition ; Nutrients ; Plant-Based Diets ; Protein .
Susan P. Himburg
Bibliography
Insel, Paul; Turner, R.; and Ross, Don (2001). Nutrition. Sudbury, MA: Jones and Bartlett.
Whitney, Eleanor N., and Rolfes, Sharon R. (2002). Understanding Nutrition, 9th edition. Belmont, CA: Wadsworth Group.

andgiant molecules as
constituents of natural
products found in plants
and animals