Much of the criticism to the Copenhagen interpretation came in the form of 'thought experiments', which could only be conducted in theory. These experiments were designed to demonstrate 'flaws' in the probabilistic interpretation, and formed the themes of intense debates. In this chapter, we shall study two such important paradoxes arising from the Copenhagen interpretation.
Schrödinger's Cat Paradox
In 1935, Schrödinger wrote a criticism of the standard view of quantum mechanics and illustrated with the example of a cat. This has since been known as the Schrödinger's cat paradox. In order to understand the concept easily, we shall explain the setup stepwise.-
Imagine a perfectly sealed box containing:
- a cat, and
- a device with a deadly poison (say potassium cyanide)
- A quantum event, namely the disintegration of a radioactive atom [1], triggers the poison device.
- There is a 50% chance that the radioactive substance decays in one hour.
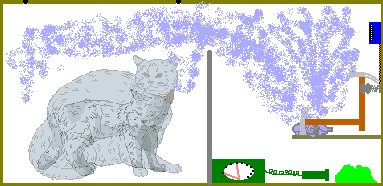
The question is: What is the state of the cat inside the box?
This whole system with the cat in the box which could be either alive or dead and the radioisotope and the poison can be described by a wave-function (in which the dead/alive states of the cat are mathematically superposed). According to Bohr, the cat is neither dead nor alive, but in an indeterminate state which is neither. The act of opening the box and looking in actually causes the wave-function to collapse into one determinate state -- dead cat or live cat. It is actually the act of observation which determines the poor cat's fate. But in reality the cat can be either dead or alive; there is no indeterminate state as such.
Einstein, Podolsky and Rosen Paradox (EPR Paradox)
Einstein attempted to demonstrate incompleteness of the standard quantum mechanical description of physical reality in a famous paper written with B. Podolsky and N. Rosen, where a thought experiment is described with persuasive reasoning.- Let us consider a particle at rest. Let us say this particle is inherently unstable, and disintegrates into two particles. (In reality, there exist particles of this kind. An example is pi-zero, which decays into two photons and has a lifetime of a tiny fraction of a second.)
- The fundamental laws of conservation, which are valid for all systems, guarantee that momentum and angular momentum [2] must be conserved. That is, the sum total of these quantities for the two particles remains the same before and after the disintegration.
- Hence we can say that the momentum and angular momentum of the two resulting particles must be equal and opposite, since before disintegration they were equal to zero.
- Therefore, measurement of the momentum of one particle can be used to deduce the value of the momentum of the partner. Alternatively one can deduce the position of the target particle with certainty and unlimited accuracy by making a position measurement on its partner.
- This trick would bypass the uncertainty principle and show that the quantum theory is incomplete.
The EPR paper was a powerful challenge to the Copenhagen group. Bohr refuted EPR by reiterating his philosophy: what is important is the whole set of conditions under which the measurement is made. He said that in the EPR scenario, the two particles form an irreducible quantum system with one wave function which incorporates both particles. Although no direct signal can travel between them, still one cannot ignore the influence of measurements on one or the other.
Einstein could never agree with this idea, calling it 'ghostly action at a distance'. But a physicist named John Bell proved in 1964 that Einstein's reality condition necessarily implies a relation among the results of a series of measurements. Numerous experiments have since been carried out to test Einstein's reality condition as well as Bell's theorem, and the results have proved that Einstein was wrong, and quantum physics was right.
Nevertheless, Bohr's Copenhagen interpretation was (and still is) not the only explanation for quantum phenomena, and even today scientists do not seem to agree on any one interpretation. In the next chapter, we shall look at some of the alternative theories proposed for quantum mechanics.
Footnotes
1. Radioactivity is a natural phenomenon in which an atom disintegrates by emitting certain particles. Since the resulting atom has a different configuration, it exhibits the properties of a different element. For example, uranium disintegrates into thorium by emitting what is called an alpha-particle. Back2. Momentum is defined as the product of the mass and velocity of a body. It is a measure of the energy of a moving body, and is zero for a body at rest. Angular momentum is a similar measure for rotating objects. Back
* Fig. 8-1 Courtesy: Tardyon.de
« Previous: Wave Mechanics || Next: Alternative Interpretations »